"The whole problem with the world is that fools and fanatics are always always certain of themselves, while wiser people are filled with doubt." - Bertrand Russell
Warning: Nerd post.
Today's nerdy topic is about Net Positive Suction Head, or NPSH from now on.
Before going any further, I should explain what NPSH is, and why it's important. People who pump liquids from place may or may not have a grasp on the subject, but they really ought to.
Net positive suction head is the amount of pressure needed at a pump's suction to keep the liquid from becoming a vapor - steam. Maybe you remember (or maybe you have forgotten) the old 8th grade science experiment where they took a glass of water at room temperature, put it in a vacuum jar, and made it boil by reducing the atmospheric pressure on it.
In the video above, the room-temperature water did not have enough heat to boil at normal air pressure. But when that air pressure was removed, the heat in the water was enough to cause it to boil briefly. Air pressure was the force that kept the molecules of water from rapidly evaporating, and once that pressure was removed, the water boiled without adding any extra heat.
Like the example above, water pumps operate on the principle of reducing pressure at the inlet to draw water into them. They mechanically create a low pressure area at the suction, which pulls water in. After the water has been pulled in, pressure is increased at the discharge. There are two common types of pumps, the centrifugal pump, and the piston pump, and both types can experience boiling at the suction.
In cases where the boiling or vaporization of water is mild, cavitation occurs. The small steam bubbles collapse again after the pump increases pressure, but the collapsing bubbles cause tiny shock waves that cause accelerated erosion in the pump. A worse case is vapor lock, when a steam bubble forms and does not quickly collapse.
Below: A pump impeller that has experienced erosion due to cavitation.
Below: Digram of a centrifugal pump. This pump operates by flinging water to the outside of the case, which creates a low pressure area in the eye of the impeller - drawing in water. The volute slows the velocity of the flung water, increasing its pressure. The eye has the lowest pressure, and is the point at which boiling is most likely to occur.
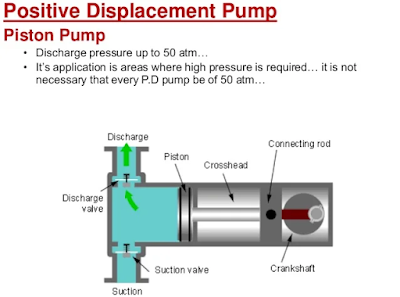
Below: A piston-driven positive displacement pump. This pump draws in liquid by increasing the volume of the cylinder as the piston moves right. This reduces pressure at the inlet and draws water in. Next the piston moves left and squeezes the pressure by reducing the volume in the cylinder. The area at the left side of the piston has the lowest pressure as the piston moves right, and is the most likely point of boiling.
With this understanding of boiling at reduced pressures, and the knowledge that pumps operate by reducing pressures, we can move on to the concept of Net Positive Suction Head. Net Positive Suction Head is the pressure required to prevent vaporizing the liquid in a pump suction. I'm going to use water, since that's the liquid most commonly pumped in the world.
Below is an old-school training video explaining how and why NPSH matters.
There are a few parameters that need to be considered besides the pressure at which room-temperature water becomes a vapor as the pressure is reduced.
- Will the water temperature always be room temperature? If the temperature of the water is higher, then more pressure on the pump suction will be required to keep it in a liquid state.
- If additional pressure is required at the pump inlet to prevent boiling, it may be necessary to provide the pump with an elevated tank on the suction to provide a column of water to provide that pressure. This column of water will be called "Static Head", and the pressure it provides will only be valid when the pump is NOT running.
- If water is to be pumped from below the level of the pump, "Static Head" must be subtracted from the available pressure.
- If fluid friction losses in the water column need to be taken into account, as a small line can starve the pump of suction pressure and also lead to boiling
- The pump internals also have flow restrictions, which reduce available NPSH.
Below is a simplified diagram explaining the points above. The pressure added by the tank above the pump adds to the overall NPSH, while the tank below subtracts from the available NPSH.
Now that you've got a better understanding of NPSH, lets take a look at some real-world examples.
Below is a water tower for municipal water system. This tank serves two purposes - it maintains NPSH on the suction of the large municipal water supply pumps, and it provides a surge volume. The surge volume is needed so that every time someone turns on or off their tap, a well water pump doesn't need to start or stop. The water level will drop down near the neck of the tank, and then a well pump will start and refill the water tower up to the top. The municipal supply pumps run all the time, and recirculate water that is not needed in homes back to the water tower.
Below: Municipal water supply pumps, for which the water tower provides NPSH.
Another real-world example, or maybe other-worldly example: There is a stage during launch of a cryogenic-liquid fueled rocket, where the engines have to be cooled down to cryogenic temperatures. This is called "Engine Chill", and again the reason is to ensure the fuel pumps for these engines have adequate NPSH.
If the piping and turbo pumps that supply fuel to the rocket engines are not pre-cooled to the temperature of the liquid cryogenic fuel, the liquid will vaporize into gas when it contacts the warm pump. The pump will then vapor-lock and pump no fuel. The launch will be a failure, the expensive pumps will be damaged by running dry.
Below: A brief test of a small turbopump with the casing removed. You get to se the pump impeller! You can see why at this kind of operating speed it would be easy to cavitate or vapor lock with cryogenic liquid in the casing.
Within a power plant, the most susceptible and expensive pump to a loss of NPSH is always the boiler feedwater pump.
Below: A boiler feedwater pump in a test setting.
Below: A small split-case boiler feedwater pump.
Below: several stacked, or "barrel-type" boiler feedwater pumps.
One reason that boiler feedwater pumps are prone to losing NPSH is that they typically are pumping pre-heated water. That is, the water has either been pre-heated by feedwater heaters and run through a steam dearator, or they are taking suction directly from the low pressure boiler drum. In either case, the water at the suction of these very expensive pumps is typically 250-350 degrees F (120-175C).
If suction pressure is ever lost on these pumps and they run dry, you can count on major, expensive damage. I've witnessed one seize while it was coasting down in a dry condition. Not a good sound...
Another reason these pumps have a tendency to lose NPSH is due to the massive volume of water that they are able to move. Large volumes of water flow increase fluid friction in the suction piping, and are also capable of rapidly emptying the tanks that supply the water.
As a result of the inherent danger or losing adequate NPSH on the boiler feedwater pumps, they are typically equipped with a pressure switch or transmitter on the suction. This switch is used to trip the motor driving the pump should the suction pressure fall low enough that boiling might occur.
Other measures can include a live NPSH calculation. When that calculated NPSH falls below a certain value, the pump can be tripped to prevent a vapor-lock condition. I prefer the calculation myself, but I've only worked one place where a live NPSH value was used for pump protection.
There's probably a lot more that could be discussed on this topic, but I've reached the end of the road on what I can remember about it!











No comments:
Post a Comment