As stated in the previous post, the Manhattan Project took a two-pronged approach to accumulating enough fissile material to build the first bomb: Uranium Enrichment and Plutonium Production.
We talked about Plutonium Production in that post. This post is about Uranium Enrichment - increasing the isotope fraction of U-235 from naturally occuring Uranium, which is mostly U-238.
Naturally occuring Uranium is 99.284% U-238, the undesirable part. Only 0.711% is U-235, the fissile stuff. The remainder is U-234, which isn't important for this discussion.
One thing is certain, you cannot accomplish this separation chemically, because U-235 and U-238 are both Uranium, and therefore they have identical chemical properties. The only technique you can use to separate the two isotopes is their weight, or more accurately, their mass.
Unfortunately, an atom of U-235 has only 1.26% less mass than an atom of U-238. Worse yet, to accomplish this separation, we need to make a compound from this Uranium and convert it into a gas first. To make it the gas requires a series of chemical reactions that begin with Uranium ore, and end with Uranium Hexafluoride (UF6) which is gaseous above about 145 degrees F. Fluorine is chosen for the compound, because it has only one stable isotope weighing 19 AMU (atomic mass units). Thus the mass of the additional Fluorine atoms will not influence the differential mass of the U-235 Hexafluoride and U-238 Hexafluoride molecules.
With the added mass of six Fluorine atoms, a U-235 Hexafluoride molecule now has only 0.852% less mass than a U-238 Hexafluoride molecule, making the difference even tinier than it was between the elemental Uranium isotopes.
Now we are ready to begin the enrichment process. The UF6, which is a gas, is heated and circulated with a compressor in a short loop. A semi-permeable membrane (made of aluminum with very minute holes) allows a fraction of the gas to escape. Here is where the magic happens: The ligher molecules escape just slightly more often than the heavy molecules do. The molecules that escape are .0043% enriched in U-235. This process is called "gaseous diffusion".
The slightly enriched gas is then the feed into another gaseous diffusion loop. A cascade of circulating loops, numbering in the thousands, each enriches the U-235 by .0043%. At the end of the cascade, we end up with a lot of depleted Uranium (that is, mostly U-238) and a little U-235. This was the technique used to create the weapons-grade Uranium that was used in the Little Boy bomb
Below is the K-25 Gaseous Diffusion plant at Oak Ridge, Tennesse. If I recall correctly, about 6000 gaseous diffusion loops were required to obtain 84% pure U-235. Considering that UF6 needs to be hot and is highly corrosive, and new lubricants for the compressors needed to be developed, this was quite an achievement for its time.
Enriched Uranium is considerably easier to make into a nuclear device than Plutonium. Greater than about 20% enrichment can be used, and there is no need for the complex trigger that a Plutonium weapon requires.
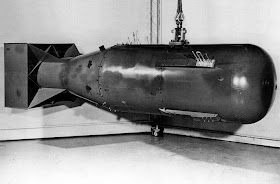
The U-235 bomb uses a "gun" type construction, with two sub-critical masses of Uranium-235 that when combined, will be highly supercritical. The gun fires a bullet of U-235 into a cup of U-235, neutrons are injected from a separate source, and the explosion follows.
If you look at the cut-away image above, and compare that with the image of the Little Boy bomb, below, you can see why an enriched Uranium bomb has the shape that it has.
Uranium is not very desirable for use in a nuclear weapon however - other than simplicity of construction. The critical mass for very highly enriched uranium is about 110 lb. To get the critical mass down to 110 lb we need about 85% enrichment. This enrichment comes at great difficulty and expense. Enormous amounts of electric power are needed to run thousands of compressors for months on end - just to make enough U-235 for a single bomb.
Meanwhile critical mass for Plutonium is just 24 lbs. Furthermore, Plutonium can be easily separated using well-understood and less energy-intensive chemical processes. Plutonium will also yield a larger explosion with less nuclear material. Due to the reduced weight of a Plutonium-based weapon, it is a much more suitable payload for a missile or aircraft to carry.
Due to the large quantity of Uranium required to make a nuclear weapon, and the enormous quantity of electricity needed to enrich the it, enriched Uranium is better suited for use in nuclear reactors than for weapons.
For the above reasons, after World War II, the gaseous diffusion Uranium Enrichment plant at Oak Ridge, Tennessee began making reactor fuels, while the Plutonium Production reactors and chemical separation plants at Oak Ridge and Hanford, Washington continued making Plutonium for weapons.
As it might be noted from recent news that there are other methods for enriching Uranium that do not involve gaseous diffusion. More modern techniques involve daisy chains of centrifuges, which are fed a constant stream of feed material, in a continuous operation rather than batch fed. As you might expect, the heavy atoms accumulate at the outside of the centrifuge, while the desirable light U-235 migrates to the center. This process is far more efficient and less energy intensive than gaseous diffusion - which is no longer in use. The centrifugal process is about 60 times more efficient than gaseous diffusion, based on the electricity required per unit of enriched Uranium produced.
There is also a very slow method that gives very high separation, and that is electromagnetic separation. It is possible to ionize (put a charge on) a Uranium atom, accelerate it, and put the elecrically charged atom in a magnetic field. A lighter U-235 atom will be deflected more than a heavy U-238 atom. As I said, this process is slow (because it's done one atom at a time) but the efficiency is high! These devices are called Calutrons, and were used during the Manhattan Project simply to have another source of U-235, however small. Calutrons were abandoned afterwards as impractical. However the sresulting U-235 was very pure and was used to increase the purity of U-235 of the gaseous diffusion plant product.
That's about all I can think of on the topic of Uranium Enrichment. Fascinating stuff!






No comments:
Post a Comment